Vastuskiri
| Dokumendiregister | Terviseamet |
| Viit | 8.1-2/24/4409-1 |
| Registreeritud | 23.04.2024 |
| Sünkroonitud | 24.04.2024 |
| Liik | Väljaminev dokument |
| Funktsioon | 8.1 Nakkushaiguste seire, ennetuse ja tõrje korraldamine |
| Sari | 8.1-2 Nakkushaiguste epidemioloogiaalane riigiväline kirjavahetus |
| Toimik | 8.1-2/2024 |
| Juurdepääsupiirang | Avalik |
| Juurdepääsupiirang | |
| Adressaat | ECDC |
| Saabumis/saatmisviis | ECDC |
| Vastutaja | Liisa Lilje (TA, Peadirektori asetäitja (2) vastutusvaldkond, Rahvatervise labor, Nakkushaiguste labor) |
| Originaal | Ava uues aknas |
Failid
Dear Pete Kinross,
Thank you for this kind reminder.
I’m gladly willing to be the contact person of Estonia for the upcoming ECDC CRAb survey.
Our OCPs will definitely be informed about the possibility to join in the upcoming network meetings in May and September.
Kind regards
Liisa Lilje
NFP AMR
Laboratory of Communicable Diseases
+372 5804 1264
Republic of Estonia
Health Board
+372 794 3500
www.terviseamet.ee
Paldiski mnt 81, 10614 Tallinn
Estonia
This e-mail is confidential and meant for use by the person named in the letterhead. Any use in any way or copying of it by a person not marked as the addressee thereof is prohibited. If you have got this e-mail by mistake, please notify of it the sender without delay and delete the received e-mail together with all its attachments.
From: Pete Kinross <[email protected]>
Sent: esmaspäev, 22. aprill 2024 17:25
To: Liisa Lilje <[email protected]>
Cc: Liidia Dotsenko <[email protected]>; Anastasia.Bilozor <[email protected]>; Liidia Dotsenko <[email protected]>; Regina Russanova <[email protected]>; TA Info <[email protected]>; Natalia Kerbo <[email protected]>; Andreas Hoefer <[email protected]>; ARHAI Meetings <[email protected]>; Dominique Monnet <[email protected]>
Subject: REMINDER (for action by Fri 26 April COB) – EE – designating a contact for ECDC CRAb survey 2024/2025; 'save the date' for CRAb survey meetings.
Importance: High
Tähelepanu! Tegemist on väljastpoolt asutust saabunud kirjaga. Tundmatu saatja korral palume linke ja faile mitte avada. |
ECDC NORMAL
Dear Liisa,
This is a reminder of the 27 March email below.
So far, 25 countries have designated a contact person for this upcoming ECDC CRAb survey. In some countries, the NFP for AMR designated themselves. Therefore, until we hear from you, we will include you as the contact person. Please let us know if you would prefer to designate a colleague instead, by close of business on Friday 26 April.
After Friday, my colleagues in [email protected] will email the invitations to the 23 May EURGen-Net virtual network meeting. For Estonia, the email will be addressed to you, with your OCPs for AMRISO in copy. You are all welcome to invite those colleagues to attend.
Participation in this online meeting does not imply an obligation for Estonia to participate in the CRAb survey. Rather, at the meeting, we will present and discuss the survey protocol, and what it means for participating countries. Here is the short version: in the ECDC CRAb survey, participating countries choose a 6-month survey period in Oct 2024–June 2025. During that survey period, laboratories/hospitals collect samples from hospitalised cases, and some associated metadata.
On 26 September, we will have a second online meeting to cover (basically) the same topics, although at that point, the survey protocol will be published.
If we can clarify, or if you would like to discuss any aspect, please do write.
Best wishes,
Pete
| |||||||||||||
|
From: Pete Kinross
Sent: Wednesday, March 27, 2024 6:35 PM
To: [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]
Cc: [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; 'Silva Tafaj (Albania)' <[email protected]>; 'Artan Bego (Albania)' <[email protected]>; 'Maja Ostojic (Bosnia and Herzegovina)' <[email protected]>; 'Arsim Kurti (Kosovo)' <[email protected]>; 'Ana Kaftandzieva (North Macedonia)' <[email protected]>; 'Deana Medic (Serbia)' <[email protected]>; [email protected]; 'Verica Jovanovic (Serbia)' <[email protected]>; 'Violeta Rakic (Serbia)' <[email protected]>; 'Baki Can Metin (Türkiye)' <[email protected]>; 'Zekiye Bakkaloglu (Türkiye)' <[email protected]>; [email protected]; Anette M. Hammerum <[email protected]>; antoni.hendrickx <[email protected]>; [email protected]; [email protected]; Orjan.Samuelsen <[email protected]>; [email protected]; [email protected]; [email protected]; Ana Rita Bastos Rebelo <[email protected]>; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; Anne-Catherine VISO <[email protected]>; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; Hanna Merk <[email protected]>; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]; International Relations <[email protected]>; Agne Bajoriniene <[email protected]>; ECDC Microbiology <[email protected]>; Andreas Hoefer <[email protected]>; ARHAI Meetings <[email protected]>; ARHAI <[email protected]>
Subject: For action by NFPs for AMR by 19 April: please designate a national contact for ECDC CRAb survey 2024/2025; 'save the date' for CRAb survey meetings.
To: ECDC National Focal Points (NFPs) for Antimicrobial Resistance (AMR); NFPs for AMR Observers;
Cc: Operational Contact Points for Microbiology - Antimicrobial-resistant isolates (AMRISO); Contact Points for Operations (CPO) for Microbiology - AMRISO; Participants from Western Balkan countries and Türkiye at the ECDC EURGen-Net network meeting (DPR179; 29–30/11/2023); Expert Group for ECDC CRAb survey; National Coordinators in Coordinating Competent Bodies; National Correspondents.
Dear NFPs for AMR and NFP for AMR Observers,
The preparations for the ECDC CRAb survey 2024/2025 are proceeding on schedule (see 15 December 2023 email below) for its start on 1 October 2024.
Thank you to everyone who emailed comments on the draft ‘survey protocol’ in December–January. Also, many thanks to the Expert Group (see email below) who first met yesterday, to discuss the draft ‘laboratory manual’. In April, we will email you the updated draft survey protocol and draft laboratory manual, for national comments.
Please can you designate a person to be a national contact for this EURGen-Net survey, by replying to this email by Friday 19 April? Preferably avoid ‘Reply all’, if possible. This person would be the main contact for survey-related emails and can be your eventual ‘National Technical Coordinator’ (NTC) for this study. As such, they should be nominated as an Operational Contact Point (OCP) for AMRISO. If they are not an OCP for AMRISO, we will gladly support you, during the summer months, with the standard nomination process ([email protected]).
Also, please can you invite up to 3 people in your country to save 2 dates in their calendar, for 2 virtual meetings for this ECDC CRAb survey? Both meetings are designed for NTCs and Reference Laboratory staff, to prepare for the start of the survey.
- ECDC EURGen-Net CRAb survey protocol meeting
Thursday 23 May 2024, from 10:00 (at earliest) until 16:00 (at latest).
This will present the pre-final survey protocol and laboratory manual. In April, will email you the draft meeting agenda, asking for your designation of ≤ 3 people. You can designate yourself. - ECDC EURGen-Net CRAb survey kick-off meeting
Thursday 26 September 2024, from 10:00 (at earliest) until 16:00 (at latest).
This is the kick-off meeting for the survey, covering FAQs.
If you have any additional questions or queries, please do email me.
Thank you again to all who already dedicated their time and expertise to this activity to prevent and control CRAb.
Best wishes,
Pete
| |||||||||||||||||||||||||||||
|
From: Pete Kinross <[email protected]>
Sent: Wednesday, 31 January 2024 17:52
To: ARHAI <[email protected]>; ECDC Microbiology <[email protected]>
Cc: ARHAI <[email protected]>; ECDC Microbiology <[email protected]>; International Relations <[email protected]>; Andreas Hoefer <[email protected]>; Pete Kinross <[email protected]>
Subject: Invitation to register by 15 Feb 2024 for ECDC Expert Group for microbiological support to ECDC survey of carbapenem-resistant Acinetobacter baumannii (CRAb)
ECDC NORMAL
To: ECDC National Focal Points (NFPs) for Antimicrobial Resistance (AMR); NFPs for Microbiology; NFPs for AMR Observers; NFPs for Microbiology Observers; Operational Contact Points for Microbiology - Antimicrobial-resistant isolates (AMRISO); Contact Points for Operations (CPO) for Microbiology - AMRISO; EARS-Net Disease Network Coordination Committee Members and Observers; Participants from Western Balkan countries and Türkiye at the ECDC EURGen-Net network meeting (DPR179; 29–30/11/2023). Cc: National Coordinators in Coordinating Competent Bodies; National Correspondents; ECDC ARHAI, ECDC Microbiology, ECDC European and International Relations.
As announced at the ECDC EURGen-Net network meeting on 29–30 November 2023, ECDC will establish an Expert Group for microbiological input to the ‘ECDC survey of carbapenem-resistant Acinetobacter baumannii (CRAb), 2024/2025’ (see * and the email below).
On 15 – 16 February 2024, ECDC will consult the ECDC Expert Directory to identify 8–15 Expert Group members. We hope that anyone interested in being part of the Expert Group registers with that Directory before then (URL: https://www.ecdc.europa.eu/en/about-ecdc/work-ecdc/external-experts). Any expert can register. It can take 5–10 minutes. Please forward this email to potentially interested colleagues.
For transparency, we have specified our search terms and selection process below this email**. If you registered in the Directory previously, we recommend that you click on the hyperlink above, to verify that your registered skills and competencies, and CV, are up-to-date.
The scope and purpose of the Expert Group for the CRAb survey is to provide advice to ECDC:
- Support production of a laboratory manual to accompany the ECDC CRAb survey protocol.
This will include (A) definition of the included species for this survey of ≤ 37 European countries; (B) specifying the best practice to prepare and send isolates and DNA; (C) providing options for the use/incorporation of nationally generated, and centrally-generated phenotypic AST data.
N.B. This will be an update of the laboratory manual for the ECDC CCRE survey, 2019. It will be shorter, as it does not include methods for double disk synergy assays, colistin AST and resistance gene specific PCRs. The 2025 survey protocol does not include a request for nationally generated WGS data, as sequencing will be performed centrally to promote data comparability.
- Minor: provide input relevant to the survey protocol itself. This minor activity will ensure consistency with the laboratory manual.
N.B. It will not include major input to the survey design, because that will occur through consultation with all eligible countries, by email and during meetings (see the email below).
- Opportunity to provide input to draft analyses in 2024/2025. Those who meet ICJME criteria will be included in a named author list in manuscript(s) for a peer-reviewed journal.
We envisage that the workload for the Group will roughly the same in 2024 (before the survey) and in 2025/2026 (during the final stages):
- 0.5–2 days of virtual meetings (including 0.25 days during the last week of March 2024); and
- 2 (max 3) email consultations on draft documents.
The timeline for the preparations is shown in the email below. The first meeting of the Expert Group is scheduled for at 13:00–16:00 CET on 26 March 2024.
If you have any questions about the above, please email To: [email protected], with Cc: [email protected].
FYI, ECDC can use the Expert Directory to identify people for a broad variety of activities, based on the competencies and preferences of the expert. For more information, please consult the Expert Directory webpage, and particularly its Q&A section.
Best wishes,
Pete Kinross (ECDC ARHAI) and Andreas Hoefer (ECDC Microbiology)
* The primary objective of the ‘ECDC CRAb survey 2024/2025’ is to describe the occurrence and geographic distribution of CRAb strains, and/or transmissible resistance/genetic elements of critical public health importance within CRAb strains, among patients in acute care hospitals in Europe, to inform prevention and control activities. The survey period is October 2024–June 2025.
** The selection process will follow these steps:
1. We will review the uploaded CVs of experts who meet the criteria below. The list of eligible terms competencies, professions and skills is purposefully broad.
2. We will identify roughly 15 people to invite in the first instance (for ‘first refusal’), based on the relevance of their expertise to the scope of the Expert Group specified above. We will specifically seek to identify experts from a diverse group of countries and expert stakeholder groups, such as EUCAST.
3. We will review the (obligatory) Declarations of Interest that are uploaded to the Directory for potential Conflicts of Interest, to ensure appropriate mitigation, according to the ECDC policy on scientific integrity and independence.
4. Subsequently, if fewer than 8 people accept, we will invite more people, in subsequent rounds, until we have 8–15 Expert Group members.
Criteria for ECDC to identify potential members of the Expert Group for ECDC CRAb survey in the ECDC Expert Directory
Profession | Biologist OR Microbiologist OR Medical doctor OR Epidemiologist |
Areas of competence | ((Area of competence = Microbiology) AND (subcategory = ‘Antimicrobial susceptibility testing’ OR ‘Anti-microbial resistance’ OR ‘Clinical microbiology’ OR ‘Molecular testing’ OR ‘Serological testing’)) OR ((Area of competence = ‘Human medicine’) AND (subcategory = ‘All’ OR Infectious diseases’ OR ‘Laboratory medicine’)) |
Specialty | (Pathogen = ‘Acinetobacter spp.’) OR (Pathogen = ‘Gram negative bacteria’) OR (Disease = ‘Acinetobacter spp. infections’) OR (Disease group = ‘Diseases caused by antimicrobial-resistant microorganisms’) |
Country | Any country (EU/EEA and non-EU/EEA countries) |
| |||||||||||||||||||||||||||||
|
From: Pete Kinross <[email protected]>
Sent: Friday, December 15, 2023 7:29 PM
To: [email protected]; [email protected]; Boudewijn.Catry <[email protected]>; ssabcheva <[email protected]>; ivanoov <[email protected]>; atambic <[email protected]>; iva.butic <[email protected]>; markella.marcou <[email protected]>; lhadjihannas <[email protected]>; helena.zemlickova <[email protected]>; vladislav.jakubu <[email protected]>; HENH <[email protected]>; Inge.Jenny.Dahl.Knudsen <[email protected]>; marina.ivanova <[email protected]>; Liisa Lilje (EE, Health Board) <[email protected]>; Jari Jalava <[email protected]>; emmi.sarvikivi <[email protected]>; [email protected]; [email protected]; sylvie.maugat <[email protected]>; EckmannsT <[email protected]>; Nolli <[email protected]>; Michalis Polemis <[email protected]>; Antonis Maragkos <[email protected]>; Kassiani Mellou <[email protected]>; tsiodras <[email protected]>; Dr. Tóth Ákos <[email protected]>; hajdu.agnes <[email protected]>; [email protected]; Anna Margrét Halldórsdóttir - Landl <[email protected]>; [email protected]; p.parodi <[email protected]>; dancona <[email protected]>; Walser-Domjan Esther <[email protected]>; Rolanda Valinteliene <[email protected]>; Monique.perrin <[email protected]>; Anne Vergison <[email protected]>; Borg Michael A at Health-MDH <[email protected]>; elizabeth.a.scicluna <[email protected]>; sabine.de.greeff <[email protected]>; annelot.schoffelen <[email protected]>; gunnar.skov.simonsen <[email protected]>; ulf.dahle <[email protected]>; Mohammed Umaer Naseer <[email protected]>; d.zabicka <[email protected]>; [email protected]; Manuela Caniça <[email protected]>; mihaela.leustean <[email protected]>; gabrielp9 <[email protected]>; eva.kendrovska <[email protected]>; [email protected]; milan.niks <[email protected]>; helena.ribic <[email protected]>; ales.korosec <[email protected]>; jesus.oteo <[email protected]>; baracil <[email protected]>; [email protected]; petra.edquist <[email protected]>; andreas.sandgren <[email protected]>
Cc: hanczvikkel.adrienn <[email protected]>; aiste.mierauskaite <[email protected]>; aiste.mierauskaite <[email protected]>; Dr. Tóth Ákos <[email protected]>; Dr. Tóth Ákos <[email protected]>; [email protected]; [email protected]; Anastasia.Bilozor <[email protected]>; ama <[email protected]>; Anna Margrét Halldórsdóttir - Landl <[email protected]>; Anna Margrét Halldórsdóttir - Landl <[email protected]>; Anna Margrét Halldórsdóttir - Landl <[email protected]>; antoni.hendrickx <[email protected]>; [email protected]; baracil <[email protected]>; Brendan Denis Kinahan <[email protected]>; Carina Brehony <[email protected]>; daan.notermans <[email protected]>; [email protected]; te-din.huang <[email protected]>; dpieridou <[email protected]>; d.zabicka <[email protected]>; e.literacka <[email protected]>; ungvari.erika <[email protected]>; Walser-Domjan Esther <[email protected]>; Walser-Domjan Esther <[email protected]>; Walser-Domjan Esther <[email protected]>; Walser-Domjan Esther <[email protected]>; etienne.lucas <[email protected]>; [email protected]; Errico Giulia <[email protected]>; Hanna.Billstrom <[email protected]>; HENH <[email protected]>; Nolli <[email protected]>; Ιωάννα Σπηλιοπούλου <[email protected]>; Isabel Cuesta De La Plaza <[email protected]>; ivanoov <[email protected]>; ivanstoikovbt <[email protected]>; Jari Jalava <[email protected]>; jaroslav.hrabak <[email protected]>; [email protected]; Jelena Razmuk <[email protected]>; jesus.oteo <[email protected]>; j.paulo.gomes <[email protected]>; João Vieira Martins <[email protected]>; henczko.judit <[email protected]>; Karl.Mertens <[email protected]>; Zacharczuk Katarzyna <[email protected]>; kati.raisanen <[email protected]>; katrien.latour <[email protected]>; [email protected]; laura.lindholm <[email protected]>; laurent.dortet <[email protected]>; Liisa Lilje (EE, Health Board) <[email protected]>; loro <[email protected]>; [email protected]; Manuela Caniça <[email protected]>; [email protected]; mjalbuquerque <[email protected]>; mperezv <[email protected]>; marianna <[email protected]>; Marie.MEO <[email protected]>; Marius Surleac <[email protected]>; [email protected]; [email protected]; monica.monaco <[email protected]>; Monique.perrin <[email protected]>; niels.pfennigwerth <[email protected]>; olivier.denis <[email protected]>; Olivier Vandenberg <[email protected]>; Orjan.Samuelsen <[email protected]>; Pedro Licinio Pinto Leite <[email protected]>; Apfalter Petra <[email protected]>; Petra Španělová <[email protected]>; Pieter-Jan Ceyssens <[email protected]>; rainer.hartl <[email protected]>; Reinis Vangravs <[email protected]>; Sinead O'Donnell <[email protected]>; solvita.selderina <[email protected]>; ssabcheva <[email protected]>; Stephan Fuchs <[email protected]>; twolkowicz <[email protected]>; semmlert <[email protected]>; Urška Kramar <[email protected]>; Vera Manageiro <[email protected]>; [email protected]; vladislav.jakubu <[email protected]>; yves.dupont <[email protected]>; PfeiferY <[email protected]>; sigrid.kiermayr <[email protected]>; NC_CCB_Austria <[email protected]>; koen.blot <[email protected]>; [email protected]; Iva Christova <[email protected]>; bernard.kaic <[email protected]>; kcapak <[email protected]>; Elisavet Constantinou <[email protected]>; Hana Orlíková <[email protected]>; [email protected]; Gideon Ertner <[email protected]>; Stine Ulendorf Jacobsen <[email protected]>; Kamilla Grønborg Laut <[email protected]>; [email protected]; natalia.kerbo <[email protected]>; Salminen Mika <[email protected]>; Otto Helve <[email protected]>; Anne-Catherine VISO <[email protected]>; Paula Garcia-Lobato <[email protected]>; Rexroth, Ute <[email protected]>; [email protected]; AnderHeidenMa <[email protected]>; Christakis Chatzichristodoulou <[email protected]>; Theodora Kalomama <[email protected]>; [email protected]; [email protected]; phc.office <[email protected]>; Rezsőfi Judit <[email protected]>; Guðrún Aspelund - Landl <[email protected]>; Greg Martin <[email protected]>; Kate Loughnane <[email protected]>; patricia.garvey <[email protected]>; Louise Cullen <[email protected]>; Lisa.Domegan <[email protected]>; kirsty.mackenzie <[email protected]>; Francesco Maraglino <[email protected]>; Antra Bormane <[email protected]>; Dehler Silvia, Dr. med. <[email protected]>; Jurgita Pakalniškienė <[email protected]>; Jurgita Pakalniškienė <[email protected]>; greta.gargasiene <[email protected]>; Jean-Claude Schmit <[email protected]>; Gauci Charmaine at Health Regulation <[email protected]>; Susan van den Hof <[email protected]>; Hester.de.Melker <[email protected]>; Macdonald, Emily Ann <[email protected]>; Poznański Dariusz <[email protected]>; Zacharczuk Katarzyna <[email protected]>; Mariana Ferreira <[email protected]>; Pedro Licinio Pinto Leite <[email protected]>; [email protected]; [email protected]; Adriana Pistol <[email protected]>; jan.mikas <[email protected]>; ecdc <[email protected]>; Marta.vitek <[email protected]>; [email protected]; [email protected]; Agneta Falk Filipsson <[email protected]>; [email protected]; [email protected]; Sara Bengtsson <[email protected]>; birgitta.lesko <[email protected]>; anette.richardson <[email protected]>; Hanna Merk <[email protected]>; Anke Kohlenberg <[email protected]>; Daniel Palm <[email protected]>; Marius Linkevicius <[email protected]>; Andreas Hoefer <[email protected]>; Pete Kinross <[email protected]>; Dominique Monnet <[email protected]>
Subject: For action by NFPs for AMR by 15 Jan 2024: comments on draft ECDC survey protocol for carbapenem-resistant Acinetobacter baumannii (CRAb)
ECDC NORMAL
To: NFPs for AMR; Cc: OCPs for AMRISO; NCs of CCBs
Dear colleagues,
Please find attached, for review, the draft ‘ECDC survey protocol for genomic-based surveillance of carbapenem-resistant Acinetobacter baumannii at the European level. Version 1.1’ . It incorporates comments and feedback received during the EURGen-Net network meeting on 30 November 2023. We welcome your feedback that you, or colleagues, may share to [email protected], by Monday 15 January 2024. Preferably use comments and/or tracked changes within the document itself. Also, if possible, please email one reply for your country.
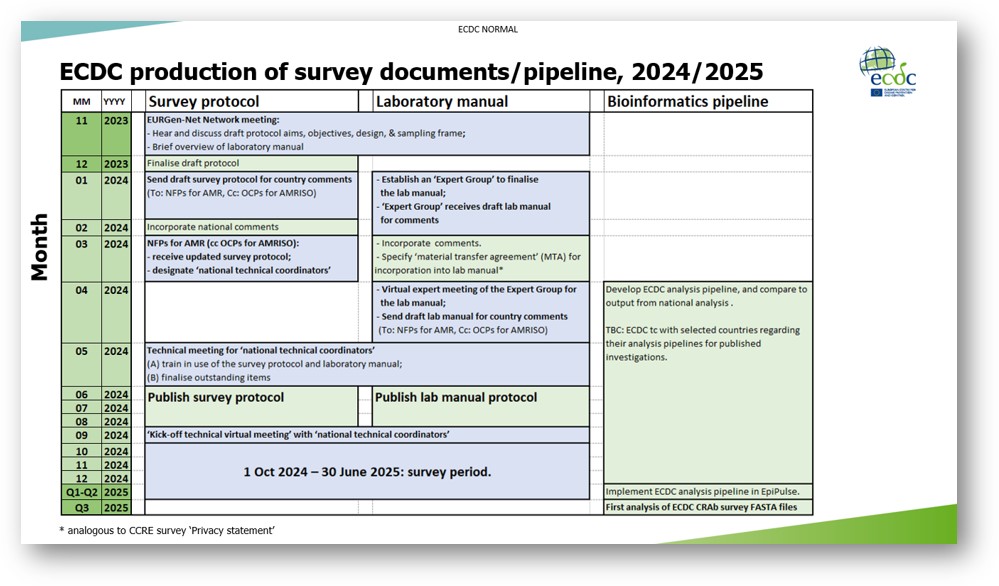
Subsequently, the survey protocol will be processed according to the timetable presented at the network meeting (please see screenshot). Specifically, in March, we will circulate an updated draft, together with the request for NFPs for AMR to designate ‘national survey coordinators’ and ‘national reference/expert laboratories’. Then, in May 2024, we will present a pre-final protocol at a technical meeting for these coordinators, to enable its publication during the summer (as ‘version 2.0’). In September, a final ‘kick-off meeting’ will focus on practical aspects for the survey (e.g. “Q&As”).
Throughout the attached draft, text in grey indicates sections that will be updated in parallel processes, such as Annexes 7 & 8. I look forward to emailing further details in the new year. For example, as presented at the network meeting (see screenshot), kindly note that a laboratory manual will be prepared in parallel, with an expert group, to cover microbiology-focussed questions. If the conclusions from that consultation impact the text of the survey protocol, the protocol and laboratory manual will be harmonised, before the May 2024 technical meeting.
Many thanks for your support to ECDC and EURGen-Net during the year, and best wishes for the festive season, for 2024, and beyond.
Kind regards,
Pete

| |||||||||||||||||||||||||||||
|
Confidentiality Notice
If you are not the intended recipient of this message, you are hereby kindly requested, to, consecutively, refrain from disclosing its content to any third party, delete it and inform its sender of the erroneous transmittal.