Kiri
| Dokumendiregister | Terviseamet |
| Viit | 11.1-2/24/11773-2 |
| Registreeritud | 13.11.2024 |
| Sünkroonitud | 14.11.2024 |
| Liik | Väljaminev dokument |
| Funktsioon | 11.1 Turustamise järgne järelevalve (post-marketing surveillance) |
| Sari | 11.1-2 Kirjavahetus Eesti turule lastavatest/kasutusele võetavatest/levitatavatest seadmetest MSA kaudu teavitamiseks |
| Toimik | 11.1-2/2024 |
| Juurdepääsupiirang | Avalik |
| Juurdepääsupiirang | |
| Adressaat | [email protected] |
| Saabumis/saatmisviis | [email protected] |
| Vastutaja | Karl Kalev Türk (TA, Peadirektori asetäitja (1) vastutusvaldkond, Meditsiiniseadmete osakond) |
| Originaal | Ava uues aknas |
Failid
From: "Meditsiiniseadmed (Medical Devices)" <[email protected]>
Sent: Thu, 03 Oct 2024 11:57:40 +0000
To: Lauryna Pudžiuvelytė <[email protected]>
Subject: Vs: Re: Change of contact person
Dear Lauryna,
Thank you for your letter.
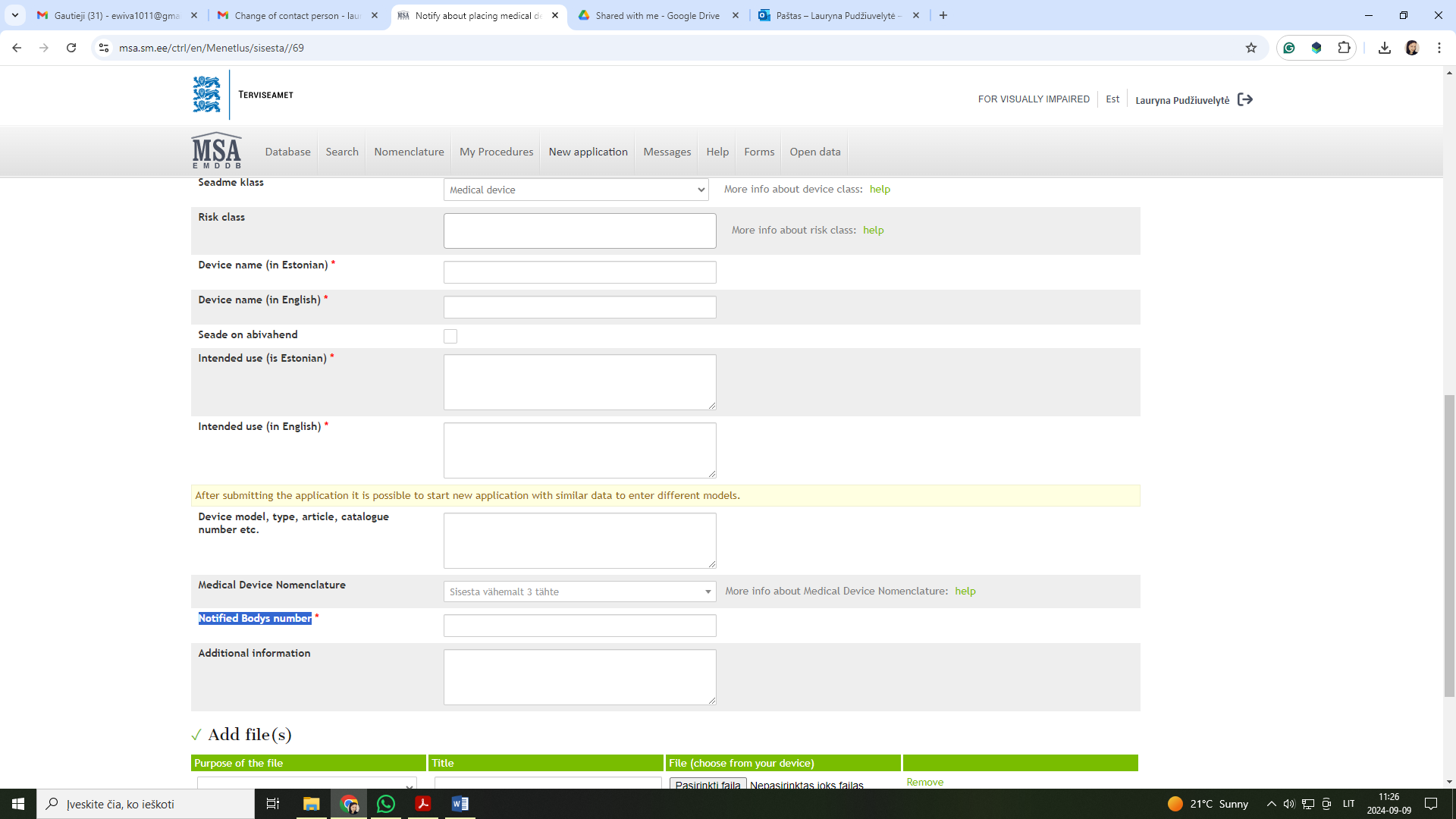
Please select the application named „Notify about medical device distribution in Estonia “. The application „Notify about placing medical device on the market“ is only for Estonia-based manufacturers or authorized representatives who are placing their medical devices on the market for the first time.
Regarding the "Notified Body number" field in the application, you may leave it blank. Although there is a red asterisk indicating that the field appears to be mandatory, it is not. You can still submit the application with this field left empty.
Do not hesitate to contact me should you require any additional information or have further questions.
Best regards,
Karl Kalev Türk
Chief Specialist
Department of Medical Devices
Phone +372 5648 5663
[email protected] | [email protected]
| Republic of Estonia Terviseamet | Health Board +372 794 3500 [email protected] Paldiski mnt 81, 10614 Tallinn Estonia |
This e-mail is confidential and meant for use by the person named in the letterhead. Any use in any way or copying of it by a person not marked as the addressee thereof is prohibited. If you have got this e-mail by mistake, please notify of it the sender without delay and delete the received e-mail together with all its attachments.
Saatja: Lauryna Pudžiuvelytė <[email protected]>
Saatmisaeg: neljapäev, 3. oktoober 2024 11:49
Adressaat: Karl Kalev Türk <[email protected]>
Teema: Fwd: [SUSPICIOUS URL INSIDE]Re: Change of contact person
Tähelepanu! Tegemist on väljastpoolt asutust saabunud kirjaga. Tundmatu saatja korral palume linke ja faile mitte avada. |
Dear Karl,
I saw that everything was OK.
So, I have a question. My company would like to place the medical devices in Estonia. So, I have to fill this field of the information: Notify about placing medical device on the market, yes?
Our medical devices are class I, but in the application form there is a mandatory place for a notified body number.

Have a nice day.
| Dr. Lauryna Pudžiuvelytė Farmacininkė, tyrėja | Pharmacist, Researcher
|
2024-09-09, pr, 11:02 TA automaatvastus <[email protected]> rašė:
Tere
Teie kiri on jõudnud Terviseameti meditsiiniseadmete osakonna e-posti aadressile [email protected]
Teile vastatakse:
• teabenõude korral 5 tööpäeva jooksul
• selgitustaotluse puhul 30 päeva jooksul
Lugupidamisega
meditsiiniseadmete osakond
Terviseamet
Dear Sir/Madam,
Medical Devices Department, Health Board has received your e-mail sent to [email protected]
Your message shall be replied:
• a request for information within 5 business days
• a request for explanation within 30 calendar days
Best regards,
Medical Devices Department
Estonian Health Board
Seosed
| Nimi | K.p. | Δ | Viit | Tüüp | Org | Osapooled |
|---|---|---|---|---|---|---|
| Kiri | 20.12.2024 | 3 | 11.1-2/24/11773-3 | Sissetulev dokument | ta | UAB" Innovative Pharma Baltics" |
| Kiri | 20.12.2024 | 3 | 11.1-2/24/11773-4 | Väljaminev dokument | ta | UAB" Innovative Pharma Baltics" |
| Kiri | 13.11.2024 | 1 | 11.1-2/24/11773-1 | Sissetulev dokument | ta | [email protected] |