Kiri
| Dokumendiregister | Terviseamet |
| Viit | 11.1-2/24/11598-5 |
| Registreeritud | 13.11.2024 |
| Sünkroonitud | 14.11.2024 |
| Liik | Väljaminev dokument |
| Funktsioon | 11.1 Turustamise järgne järelevalve (post-marketing surveillance) |
| Sari | 11.1-2 Kirjavahetus Eesti turule lastavatest/kasutusele võetavatest/levitatavatest seadmetest MSA kaudu teavitamiseks |
| Toimik | 11.1-2/2023 |
| Juurdepääsupiirang | Avalik |
| Juurdepääsupiirang | |
| Adressaat | Abbott Laboratories Poland |
| Saabumis/saatmisviis | Abbott Laboratories Poland |
| Vastutaja | Karl Kalev Türk (TA, Peadirektori asetäitja (1) vastutusvaldkond, Meditsiiniseadmete osakond) |
| Originaal | Ava uues aknas |
Failid
From: "Meditsiiniseadmed (Medical Devices)" <[email protected]>
Sent: Fri, 19 May 2023 06:56:13 +0000
To: "Adamczyk; Emilia" <[email protected]>
Cc: RegulatoryEUEFTA <[email protected]>
Subject: Vs: Request for clarification_IVD notification by Distributor
Dear Emilia,
Indeed, every distributor who distributes devices in Estonia (which are subject to notification due to the risk class) must notify us.
And yes, every distributor even when they do not add documents (will not be asked if the existing documents in the database are valid) will receive a PDF document, stating that the device X have been added to the Estonian Medical Devices and Appliances Database.
It also contains clarification that this is not a validation or approval, e.g. the following is an extract from such a confirmation:

Best regards,
Karl Kalev Türk
Chief Specialist
Department of Medical Devices
Phone +372 5648 5663
[email protected] | [email protected]
| Republic of Estonia Terviseamet | Health Board +372 794 3500 [email protected] Paldiski mnt 81, 10614 Tallinn Estonia |
This e-mail is confidential and meant for use by the person named in the letterhead. Any use in any way or copying of it by a person not marked as the addressee thereof is prohibited. If you have got this e-mail by mistake, please notify of it the sender without delay and delete the received e-mail together with all its attachments.
Saatja: Adamczyk, Emilia <[email protected]>
Saatmisaeg: esmaspäev, 15. mai 2023 16:55
Adressaat: Meditsiiniseadmed (Medical Devices) <[email protected]>
Koopia: RegulatoryEUEFTA <[email protected]>
Teema: RE: Request for clarification_IVD notification by Distributor
Tähelepanu! Tegemist on väljastpoolt asutust saabunud kirjaga. Tundmatu saatja korral palume linke ja faile mitte avada. |
Dear Karl,
Thank you very much for your prompt replay and clarification.
We were asking for the clarification as in regulation is used sentence “first distribution” and we were not sure if it is first distribution of the product in Estonia, or first distribution of the product made by every distributor.
Regarding § 5 (2) – I was referring to the “Tellimusmeditsiiniseadme turul kättesaadavaks tegemisest ning sellel tehtud olulisestmuudatusest ja meditsiiniseadme levitamisest teavitamise tingimused” ( see attached).
Could you please confirm if according § 5 (2) mentioned above every distributor who submitted notification can receive notification certificate issued by the Health Board? Even if this distriubutor notify Agency as a second and does not need to submit all the documentation?
Best regards/Pozdrawiam,
Emilia
| |||||||||||||||||||||
This communication may contain information that is proprietary, confidential, or exempt from disclosure. If you are not the intended recipient, please note that any other dissemination, distribution, use or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from his or her computer.
From: Meditsiiniseadmed (Medical Devices) <[email protected]>
Sent: piątek, 12 maja 2023 09:59
To: Adamczyk, Emilia <[email protected]>
Cc: RegulatoryEUEFTA <[email protected]>
Subject: Vs: Request for clarification_IVD notification by Distributor
EXTERNAL EMAIL: Only click links or open attachments if you recognize the sender and know the content is safe. |
Dear Emilia,
Thank you for your inquiry. Unfortunately, we have not received your previous email, and as a result, it has remained unanswered.
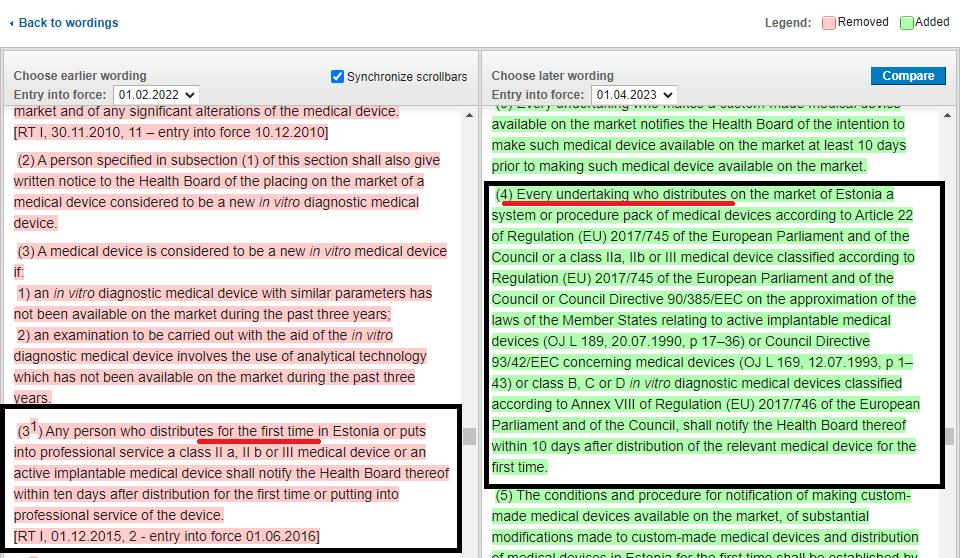
Indeed, Estonian Medical Device Act changed in 1.01.2023. The new version changed the principle of informing about the distribution of devices, meaning now all distributors have obligation to notify about distribution of devices. Previously, only the first distributor had to inform about the distribution.
I will also highlight the comparison between the old and the new version of the law regarding notifying about the distribution of devices.

In this regard, we have also simplified the procedure for informing about distribution. Specifically, subsequent distributors no longer need to add a complete set of documents to the application, provided that it is not an old entry and the public documents are the same. In this case, it should be indicated in the explanation field of the application that the public documents are the same. However, the processor of the application still has the right to request a complete set of documents (for example, if non-public documents, such as the Declaration of Conformity or EC-certificate, have expired).
Could you please clarify your last question? I'm currently not understanding which regulation/law § 5 (2) you are referring to, where both IVDs and custom-made devices are included.
Best regards,
Karl Kalev Türk
Chief Specialist
Department of Medical Devices
Phone +372 5648 5663
[email protected] | [email protected]
| Republic of Estonia Terviseamet | Health Board +372 794 3500 [email protected] Paldiski mnt 81, 10614 Tallinn Estonia |
This e-mail is confidential and meant for use by the person named in the letterhead. Any use in any way or copying of it by a person not marked as the addressee thereof is prohibited. If you have got this e-mail by mistake, please notify of it the sender without delay and delete the received e-mail together with all its attachments.
Saatja: Adamczyk, Emilia <[email protected]>
Saatmisaeg: reede, 12. mai 2023 10:19
Adressaat: Meditsiiniseadmed (Medical Devices) <[email protected]>
Koopia: RegulatoryEUEFTA <[email protected]>
Teema: FW: Request for clarification_IVD notification by Distributor
Tähelepanu! Tegemist on väljastpoolt asutust saabunud kirjaga. Tundmatu saatja korral palume linke ja faile mitte avada. |
Dear All,
Could you please be so kind and respond for email below? We will be very grateful for your help.
Best regards/Pozdrawiam,
Emilia
| |||||||||||||||||||||
This communication may contain information that is proprietary, confidential, or exempt from disclosure. If you are not the intended recipient, please note that any other dissemination, distribution, use or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from his or her computer.
From: RegulatoryEUEFTA
Sent: czwartek, 6 kwietnia 2023 12:22
To: Meditsiiniseadmed (Medical Devices) <[email protected]>
Subject: Request for clarification_IVD notification by Distributor
Dear All,
We would like to ask you for clarification § 26(4) of Medical Devices Act which is as follows:
(4) Any economic operator distributing on the Estonian market a system or procedure pack of medical devices pursuant to Article 2017 of Regulation (EU) 745/22 of the European Parliament and of the Council or Regulation (EU) 2017/745 of the European Parliament and of the Council or Council Directive 90/385/EEC on the approximation of the laws of the Member States relating to active implantable medical devices (OJ L 189, 20.07.1990, p. 17–36) or Council Directive 93/42/EEC concerning medical devices (OJ L 169, 12.07.1993, pp. 1-43), a medical device classified according to risk class IIa, IIb or III, or an in vitro diagnostic medical device classified as class B, C or D in vitro diagnostic medical device in accordance with Annex VIII to Regulation (EU) 2017/746 of the European Parliament and of the Council, shall notify the Health Board thereof within ten days of the first distribution of the medical device concerned.
If an IVD (Class B, C, D) is distributed in Estonia by two entities: an Estonian distributor and by a distributor from outside Estonia – do both Distributors need to notify Estonian Agency about distributing this IVD on the Estonian market? Or does this product need to be notified once –only by the distributor who performed first distribution of the IVD on the Estonian market?
If it is sufficient that only one distributor notifies about the IVD, does the certificate issued according to the § 5 (2) of regulation Conditions for the notification of the making available on the market of a custom-made device and of any substantial modification thereto and of the distribution of a medical device cover the activity of the other distributor who did not perform the notification?
Thank you,
Emilia Adamczyk

Seosed
| Nimi | K.p. | Δ | Viit | Tüüp | Org | Osapooled |
|---|---|---|---|---|---|---|
| Kiri | 13.11.2024 | 1 | 11.1-2/24/11598-1 | Sissetulev dokument | ta | Abbott Laboratories Poland |
| Kiri | 13.11.2024 | 1 | 11.1-2/24/11598-2 | Väljaminev dokument | ta | Abbott Laboratories Poland |
| Kiri | 13.11.2024 | 1 | 11.1-2/24/11598-3 | Sissetulev dokument | ta | Abbott Laboratories Poland |
| Kiri | 13.11.2024 | 1 | 11.1-2/24/11598-4 | Sissetulev dokument | ta | Abbott Laboratories Poland |
| Kiri | 13.11.2024 | 1 | 11.1-2/24/11598-6 🔒 | Väljaminev dokument | ta | Abbott Laboratories Poland |