Vastuskiri
| Dokumendiregister | Terviseamet |
| Viit | 11.1-2/24/1712-2 |
| Registreeritud | 13.02.2024 |
| Sünkroonitud | 27.03.2024 |
| Liik | Väljaminev dokument |
| Funktsioon | 11.1 Turustamise järgne järelevalve (post-marketing surveillance) |
| Sari | 11.1-2 Kirjavahetus Eesti turule lastavatest/kasutusele võetavatest/levitatavatest seadmetest MSA kaudu teavitamiseks |
| Toimik | 11.1-2/2023 |
| Juurdepääsupiirang | Avalik |
| Juurdepääsupiirang | |
| Adressaat | Baltics Medical |
| Saabumis/saatmisviis | Baltics Medical |
| Vastutaja | Karl Kalev Türk (TA, Peadirektori asetäitja (1) vastutusvaldkond, Meditsiiniseadmete osakond) |
| Originaal | Ava uues aknas |
Failid
From: "Meditsiiniseadmed (Medical Devices)" <[email protected]>
Sent: Fri, 24 Nov 2023 06:29:39 +0000
To: Rasa Pudžaitienė <[email protected]>
Subject: Vs: instructions how a distributor can add the manufacturer
Dear Rasa,
Thank you for the inquiry.
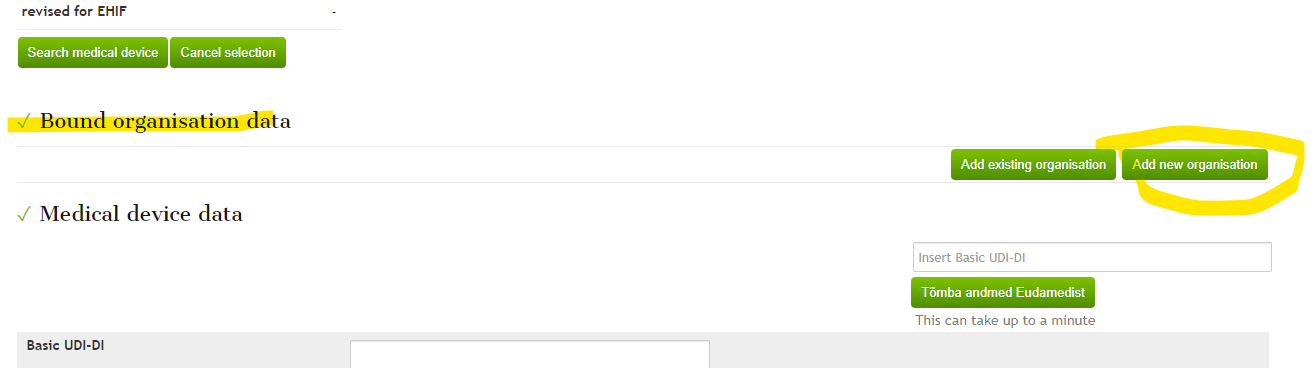
When distributing a device whose manufacturer is not already in the database, you can add the manufacturer's details when filling out the „Notify about medical device distribution in Estonia“ application in the „Bound organisation data“ block by using the „add new organization“ button. Select the role as the manufacturer for the entered data.

Please do not hesitate to contact me for further information or with any questions.
Best regards,
Karl Kalev Türk
Chief Specialist
Department of Medical Devices
Phone +372 5648 5663
[email protected] | [email protected]
| Republic of Estonia Terviseamet | Health Board +372 794 3500 [email protected] Paldiski mnt 81, 10614 Tallinn Estonia |
This e-mail is confidential and meant for use by the person named in the letterhead. Any use in any way or copying of it by a person not marked as the addressee thereof is prohibited. If you have got this e-mail by mistake, please notify of it the sender without delay and delete the received e-mail together with all its attachments.
Saatja: Rasa Pudžaitienė <[email protected]>
Saatmisaeg: neljapäev, 23. november 2023 09:03
Adressaat: Meditsiiniseadmed (Medical Devices) <[email protected]>
Teema: instructions how a distributor can add the manufacturer
Tähelepanu! Tegemist on väljastpoolt asutust saabunud kirjaga. Tundmatu saatja korral palume linke ja faile mitte avada. |
Dears,
We would like to notify you about the distribution of a medical device in Estonia. However, the manufacturer is not currently listed in the Estonian database.
Could you please provide me with step-by-step instructions on how a distributor can add the manufacturer to the database.
Thank you in advance.
Rasa Pudžaitienė
Public Procurement Specialist
M: +37065767869
![]()
Seosed
| Nimi | K.p. | Δ | Viit | Tüüp | Org | Osapooled |
|---|---|---|---|---|---|---|
| Vastuskiri | 13.02.2024 | 43 | 11.1-2/24/1712-3 🔒 | Sissetulev dokument | ta | Baltics Medical UAB |
| Kiri | 13.02.2024 | 43 | 11.1-2/24/1712-1 | Sissetulev dokument | ta | Baltics Medical |
| Vastuskiri | 13.02.2024 | 43 | 11.1-2/24/1712-4 🔒 | Väljaminev dokument | ta | Baltics Medical UAB |