Selgitustaotlus
| Dokumendiregister | Terviseamet |
| Viit | 10.2-6/25/302-3 |
| Registreeritud | 21.05.2025 |
| Sünkroonitud | 22.05.2025 |
| Liik | Sissetulev dokument |
| Funktsioon | 10.2 Toodete terviseohutusega seotud toimingud |
| Sari | 10.2-6 Teabenõuded, selgitustaotlused, märgukirjad toodete ohutuse valdkonnas |
| Toimik | 10.2-6/2025 |
| Juurdepääsupiirang | Avalik |
| Juurdepääsupiirang | |
| Adressaat | S-ilu OÜ/ VegePure |
| Saabumis/saatmisviis | S-ilu OÜ/ VegePure |
| Vastutaja | Marina Karro (TA, Peadirektori asetäitja (1) vastutusvaldkond, Kemikaaliohutuse osakond) |
| Originaal | Ava uues aknas |
Failid
Date: 03.20.2025. v3
1/6
SAFETY DATA SHEET CYCLE TOILET CLEANER LAVENDER-MINT 500 ml, 3000 ml, 10 l
Produced under Regulation 1272/2008/EC and 648/2004/EC
SECTION 1: Identification of the mixture and the company
1.1. Product ID: CYCLE Toilet cleaner lavender-mint
1.2. Identified use: Cleaning of tiles, toilet. Lime scale remover.
User advice: Do not apply on marble and copper or any other acid sensitive surface.
1.3. Manufacturer:
Renew Technologies Hungary Kft.
Address: 1139 Budapest, Lomb u. 15.
Phone: +36 70 474 9900
email: [email protected]
1.4. Emergency telephone:
Health & Toxicology Department in your country
Company phone number in case of emergency (24/7): +36 20 920 4608
SECTION 2: Hazards identification
2.1 Classification of the mixture
2.2. Label elements:
Not a hazardous mixture.
Components: 1-10% Citric acid; 1-10% Non-ionic surfactants, <1% essential oils
Application: Apply undiluted, leave it on the surface for a few minutes, then wipe it off. Do not use on acid-
sensitive surfaces (e.g. marble, copper)! In case of contact with eyes, rinse immediately with water! If using
contact lenses, remove them and continue rinsing!
Storage: in original, sealed packaging, between 10-30 °C, protected from direct sunlight, out of reach of
children.
Best before: 3 years from production. See the date of production on the bottle.
2.3. Other hazards:
PBT: None
vPvB: None
If the mixture is used properly, it does not harm the environment.
The mixture is not fire and explosion hazard.
SECTION 3: Composition data
3.1. Mixture:
Date: 03.20.2025. v3
2/6
Substance/Mixture name
EC number % by weight Classification according to Regulation (EC) No 1272/2008
Spec. conc. limits, M-factors and ATEs
D-Glucopyranose, oligomers, decyl, octyl glycosides, aqueous solution
500-220-1 1-10% - -
Citric acid 201-069-1 1-10% Eye Irrit. 2, H319 STOT SE 3, H335
-
Lavender oil (Natural Oil)
616-770-1 <1% - -
Mentha arvensis extr.
290-058-5 <1% - -
For full text of Hazard and EU Hazard-statements: see SECTION 16
SECTION 4: First aid measures
4.1. Description of first aid measures:
In case of eye contact: In case of contact with the eyes, rinse immediately with water. If using contact
lenses, remove them and continue rinsing.
In case of skin contact: No need for special emergency measures.
In case of ingestion: Never give an unconscious person anything by mouth. The injured person should
drink as much water as she/he can.
4.2. Most important - acute and delayed - symptoms and effects: Not considered to be hazardous.
4.3. The immediate medical attention and special treatment: No need for special treatment. Note to Physician:
Treat according to symptoms.
SECTION 5: Fire-fighting measures
5.1. Extinguishing agent: Normal extinguishing agents used: carbon dioxide, sand, water spray for large fires,
firefighting foam.
5.2. Special hazards arising from the substance or mixture: Not known.
5.3. Advice for fire-fighters: Not known.
SECTION 6: Accidental release measures
6.1. Personal precautions, protective equipment and emergency procedures
For non-emergency personnel: Use personal protection (see in Section 8.)
For emergency responders: Use proper personal protection (see in Section 8.)
6.2. Environmental precautions
Large amounts of spillage materials should not get into the sewage system, water bodies!
6.3. Containment and cleaning up methods and materials
Spills should be neutralized with water. Absorbent material (eg. cloth, fleece) should be used for wiping.
A small amount of product must be removed according to the usual cleaning procedure.
6.4. Reference to other sections: See Section 7. for handling and storage information. For personal protection see
Section 8. Waste management information, see Section 13.
Date: 03.20.2025. v3
3/6
SECTION 7: Handling and storage
7.1. Precautions for safe handling: Contact with eyes and igestion should be avoided.
7.2. Conditions for safe storage, including any incompatibilities: The product must be stored between 10-30℃.
Do not expose it to direct heat or sunlight. No restrictions on storage together with other products. Keep out of reach
of children! Always use the original labelled packaging.
7.3. Product use: Cleaning of tiles, toilet. Lime scale remover.
SECTION 8: Exposure controls/personal protection
8.1. control parameters
No occupational exposure limit for ingredients.
8.2. Exposure monitoring
Respiratory protection: No special requirements
Hand protection: No special requirements
Eye protection: No special requirements
Skin and body protection: No special requirements
Hygiene measures: Handle according to good industrial hygiene and safety practice.
Wash hands before breaks and after work.
Environmental exposure: Does not harm the environment under intended use, transport and storage.
SECTION 9: Physical and chemical properties
9.1. Information on basic physical and chemical properties:
General Information
Appearance (physical state): liquid
Colour: seethrough, opalesque
Smell: flowery, with acidic notes
Important health, safety and environment related information:
pH: 2,0-2,5
Melting point/range: no data
Boiling point: no data
Inflammation point: no data
Flash point: no data
Flammability: not flammable
Explosion hazard: not explosive
Explosion limits: no data
Decomposition temperature: no data
Pour point: no data
Relative density: 1.0-1.1 g/cm3 (at room temperature)
Water solubility: soluble in water
Viscosity: water-like
Oxidizing properties: no data, not typical
9.2. Other information: no data, not typical or not available
Date: 03.20.2025. v3
4/6
SECTION 10: Stability and reactivity
10.1. Reactivity: The mixture is not reactive.
10.2. Chemical stability: At room temperature and normal pressure conditions the mixture is chemically
stable.
10.3. Possibility of hazardous reactions: If used properly, no dangerous reaction takes place.
10.4. Conditions to avoid: Direct sunlight, high temperatures and frost.
10.5. Incompatible materials: Not known.
10.6. Hazardous decomposition products: If used properly, no hazardous decomposition products are formed.
SECTION 11: Toxicological information
11.1. Information on toxicological effects: Product toxicology studies have not been performed, toxicological
assessment made solely on the basis of data on the individual components.
11.1.1. The toxicity data of the ingredients:
Name Acute toxicity,
oral (mg/ttkg)
Acute toxicity, dermal
(mg/ttkg)
Acute toxicity, inhalation
(mg/l)
Citric acid 5400 1 >2000 1 75 2
D-Glucopyranose, oligomers, decyl octyl glycosides
>2000 1 20002 n.d.
Lavender oil >2000 1 >2000 1 20 1
Mint oil >2000 1 >5000 2 n.d.
1 derived from MSDS
2 derived from ECHA database
11.1.2. The symptoms after the exposure of the mixture:
Inhalation: Not known.
Ingestion: Not known.
Skin irritation: Not known.
Eye irritation: Not known.
Sensitivity: Not known.
Sensitization: Not known
Repeated/prolonged exposure: Not known
Carcinogenicity: Not known.
Mutagenesis: Not known
SECTION 12: Ecological information
12.1. Toxicity: Tests have not been performed. The classification and concentration of the ingredients were
determined according to the CLP Regulation requirements and on the basis of toxicological data.
Date: 03.20.2025. v3
5/6
Name EC50 (mg/l)
LC50 (mg/l)
PNEC (g/l)
Citric acid n.d. 440 1 no hazard 2
D-Glucopyranose, oligomers, decyl octyl glycosides
>100 2 126 2 0,176 2
Lavender Oil n.d. n.d. 0,024 2
Mint Oil n.d. n.d. n.d.
1 derived from MSDS 2 derived from ECHA database 12.2. Stability and degradability: biodegradable
12.3. Bioaccumulation potential: no data
12.4. Mobility in soil: it has good solubility and low adsorption capacity in soil.
12.5. PBT assessments: not PBT or vPvB
12.6. Other adverse effects: no data.
SECTION 13: Disposal considerations
13.1. Waste treatment methods: The product residues and wastes must be treated according to the national
regulations of your country.
SECTION 14: Transport Information
The product is according to the control of international transport of dangerous goods agreements (ADR/RID, IMDG,
IATA/ICAO): NOT DANGEROUS.
SECTION 15: Regulatory information
15.1. Safety, health and environmental regulations/legislation for the substance or mixture
Relevant European Community legislation:
CLP Decree: 1272/2008/EC and its amendments; REACH Decree: 1907/2006/EC and its amendments;
Detergent Decree: 648/2004/EC and its amendments
Occupational exposure limits: 91/322/EC Decree and it modification; 2000/39/EC Directive and its
amendments
Workplace safety: 1993. évi XCIII. Act on occupational safety and health; 25/2000. (IX.30.) EüM-SzCsM
Decree about the chemical safety of workplaces; 33/1998. (VI.24.) NM Decree on medical examination and
opinion about job and personal hygiene adequacy; 3/2002. (II.8.) SzCsM-EüM Decree on the minimum health
and safety requirements in workplaces.
Chemical Safety: 2000. évi XXV. Act on chemical safetly and its amendments, certain proceedings related to
hazardous materials and hazardous products furthermore the related activities are regulated in 44/2000.
(XII.27.) EüM Decree and its amendments.
Date: 03.20.2025. v3
6/6
Environment: 1995. évi LIII. Act on the general rules of environmental protection; 2012. évi CLXXXV. Act on
wastes; 98/2001. (VI.15.) Government Decree on activities related to hazardous waste; 72/2013. (VIII.27.)
VM Decree on list of waste
Fire protection: 1996. évi XXXI. Act on fire protection, the technical rescue and fire departments; 54/2014.
(XII.5.) BM Decree on National Fire Protection Regulations
15.2. Chemical safety assessment: No Chemical Safety Assessment has been performed for the mixture.
SECTION 16: Other information
The MSDS is valid for the delivered state of the product. The safety data sheet is only describing the product based
on safety requirements and is not intended to guarantee certain properties and does not replace the product
specifications.
The Material Safety Data Sheet has been compiled according to the best of our knowledge, to the basis of the material
safety data sheets of ingredient manufacturers, the relevant laws, regulations and literature. The rules and regulations
in force are obligatory for the users.
The user is responsible for the appropriate use of the product. The Material Safety Data Sheet does not undertake any
responsibility or legal liability about the use of the product in any circumstances or the results of improper use. The
circumstances of the use (handling, use, storage, disposal, etc) are beyond our competence.
H-phrases of the mixture: None.
H-phrases used in the safety data sheet in Chapter 3.:
H 335: May cause respiratory irritation.
H 319: Causes serious eye irritation.
Do not mix with other chemicals or detergents!
Tere
Palun registreerida ja saata mulle.
Ette tänades
Marina Karro
Saatja: [email protected] <[email protected]>
Saatmisaeg: neljapäev, 15. mai 2025 16:12
Adressaat: Marina Karro <[email protected]>
Teema: [SUSPICIOUS URL INSIDE]Re: Küsimused
Tähelepanu! Tegemist on väljastpoolt asutust saabunud kirjaga. Tundmatu saatja korral palume linke ja faile mitte avada. |
Tere
Kõige pealt tahan ma tänada nii põhjaliku ülevaate eest! Vabandust, et ma varem ei vastanud, kuna ma töötasin läbi kõiki asju ja see võttis aega.
Igatahes olen ikka veel veidi segaduses. Ma saadan teile ühe ohutuskaardi ühe toote kohta.
Kas saan õigesti aru, et pean selle eesti keelde tõlkima? Ohutuskaardil peab olema levitaja info. Kas see tähendab, et pean lisama 1.3 alla enda kontaktandmed? Punktis 1.4 peavad olema Eesti hädaabitelefonide numbrid välja toodud?
Grossi Toidukaupade ostujuht mainib, et peaksin Terviseametiga kõik ennem kooskõlastama.
Kõike head
Signe Õunapuu
On Thu, Apr 17, 2025 at 10:06 AM Marina Karro <[email protected]> wrote:
Tere
Puhastusvahendid peavad vastama detergendimääruse nõuetele (Artikkel 11- märgistus; biolagunevuse nõuded), CLP-määrusest (märgistus, mürgistusteabekeskuse teavitamine, ohtlikest koostisainetest teavitamine) ja vajadusel peab olema koostatud REACH-määruse II lisa nõuetele vastav ohutuskaart. Ohutuskaart ja märgistus peavad olema eestikeelsed.
Alates 1. jaanuarist 2025 peab CLP-määruse I lisa kohaselt ohtlikuks klassifitseeritud segude märgistusel (pakendil) olema UFI-kood ning tehtud teavitus sellistest segudest Euroopa Kemikaaliameti mürgistusteabekeskuste portaali kaudu (ECHA PCN Portal). Teavitamisega kaasneb UFI-koodi (unikaalne koostise tähis) kasutuselevõtmine. See on tähtede ja numbrite kombinatsioon, mis on seotud ainult konkreetse seguga.
Kui need puhastus- ja pesemisvahendid ei ole ohtlikuks klassifitseeritud, peab olema koostatud koostisosade andmeleht vastavalt detergendimääruse artiklile 9(3).
Palun tutvuge terviseameti veebilehel oleva infoga:
Detergendid | Terviseamet, Ohutuskaart | Terviseamet.
Vastuseks Teie teisele küsimusele teatan, et seebid kuuluvad kosmeetikatoodete hulka.
Kosmeetikatoode on aine või segu, mis on ette nähtud kokkupuuteks inimese keha välispinna osadega (nahk, juuksed, näo- ja ihukarvad, küüned, huuled ja välised suguelundid) või hammaste ja suuõõne limaskestadega ainult või peamiselt nende puhastamiseks, lõhnastamiseks, nende välimuse muutmiseks, nende kaitsmiseks, heas seisundis hoidmiseks või ihulõhnade parandamiseks. Kosmeetikal ei ole lubatud kasutada meditsiinilisi ehk ravivaid väiteid.
Kosmeetikatoodete kohta leiate informatsiooni Terviseameti koduleheküljel kemikaali ja tooteohutuse rubriigist aadressil Kosmeetikatooted – info ettevõtjale | Terviseamet.
Kosmeetikatooteid reguleerib rahvatervise seadus, Euroopa Parlamendi ja Nõukogu määruse nr 1223/2009/EÜ kosmeetikatoodete kohta ja Komisjoni määrus nr 655/2013/EL kosmeetikatoodetel esitatavate väidete ühtsete nõuete kohta.
Kuna Teie valmistate kosmeetikatooteid, siis antud juhul Teile rakenduvad kosmeetikamääruses sätestatud tootja kohustused.
Nõuded kosmeetikatoodete tootmisele (sh ruumidele) on toodud standardis EVS-EN ISO 22716:2008 „Kosmeetikatooted. Head tootmistavad. Juhised heade tootmistavade osas“, mille saate endale soetada Eesti standardikeskuse e-poes aadressil. See on harmoneeritud standard. Standardis on toodud kõik nõuded ruumidele, tootmisele, personalile, dokumentatsioonile jne. Kosmeetikatoodetel peab olema kindel retseptuur ja tootmisel peab olema juurutatud hea tootmistavale vastav kvaliteedisüsteem. Siin tuleb silmas pidada et kosmeetikatoodete valmistamiseks ruumid peavad vastama standardi nõuetele, näiteks kodus või muul otstarbel kasutatavates ruumides kosmeetika tootmine ei ole aktsepteeritav. Kosmeetikatoodete ohutus peab olema kindlaks tehtud.
Kosmeetikatoodete tootmiseks Terviseametilt kooskõlastust ega luba ei ole vaja. Mingit eelnevat dokumentide esitamist Terviseametile seadusandlusega ei ole ette nähtud. Kui Te kavatsete tootma hakata kosmeetikatooteid olete te kosmeetikamääruse mõistes tootja, ehk vastutav isik. Eesti äriregistris peab Teil olema registreeritud kas ettevõte või FIE.
Vastutava isiku kohustused on toodud kosmeetikamääruse artiklis 5 p.1:
Vastutav isik tagab vastavuse artiklitega 3 (Ohutus), 8 (Hea tootmistava), 10 (Ohutushinnang), 11 (Toote andmik), 12 (Proovide võtmine ja analüüs), 13 (Teavitamine), 14 (Lisades loetletud ainete piirangud), 15 (Kantserogeenseks, mutageenseks või reproduktiivtoksiliseks aineks klassifitseeritud ained), 16 (Nanomaterjalid), 17 (Keelatud ainete jäljed), 18 (Loomkatsed), artikli 19 (Märgistamine) lõigetega 1, 2 ja 5, artiklitega 20 (Väited tootel), 21 (Üldsuse juurdepääs teabele), 23 (Tõsisest soovimatust mõjust teatamine) ja 24 (Andmed ainete kohta).
Vastutaval isikul iga toodetud kosmeetikatoote kohta peab olema koostatud toote andmik koos kosmeetikatoote ohutusearuandega, kus on kosmeetikatoote ohutus hinnatud vastava ohutusehindaja poolt ja iga kosmeetikatoote kohta peab vastutav isik teavitama CPNP teavitamise portaalis. Teavitamise protseduur on tasuta. Teavitamise protseduur on reguleeritud kosmeetikamääruse artikliga 13. Põhjaliku infoga ja juhisega kuidas veebipõhiselt teavitamisprotseduuri täita saate tutvuda Terviseameti koduleheküljel Kosmeetikatooted – info ettevõtjale | Terviseamet Samal aadressil artiklis leiate ka juhised CPNP teavitamise kohta. CPNP - kosmeetikatoodete teavitamise portaal koos piltliku õpetusega asub aadressil https://webgate.ec.europa.eu/cpnp/
Teil, kui tootjal peab olema toote andmik, mida säilitatakse kümme aastat pärast kuupäeva, mil kosmeetikatoote viimane partii turule lasti ning see peab olema kergesti kättesaadav liikmesriigi pädevale asutusele.
Toote andmik sisaldab järgmist teavet ja andmeid, mida vajaduse korral ajakohastatakse:
a) kosmeetikatoote selline kirjeldus, mis selgesti näitab, et toote andmik kuulub selle toote juurde;
b) artikli 10 lõikes 1 osutatud kosmeetikatoote ohutusaruanne (ohutusaruande koostamise üksikasjad on toodud kosmeetikamääruse lisas I ja Komisjoni rakendusotsus 2013/674/EL, 25. november 2013, Euroopa Parlamendi ja nõukogu määruse (EÜ) nr 1223/2009 (kosmeetikatoodete kohta) I lisa käsitlevate suuniste kohta);
c) tootmismeetodi kirjeldus ja kinnitus selle kohta, et tootmisel on järgitud artiklis 8 osutatud head tootmistava;
d) tõendid kosmeetikatoote väidetava mõju kohta, kui see on kosmeetikatoote laadi või mõju tõttu põhjendatud;
e) andmed tootja, tema esindajate või tarnijate poolt läbiviidud loomkatsete kohta seoses kosmeetikatoote või selle koostisainete väljatöötamise või ohutushinnanguga, sealhulgas loomkatsed, mis on läbi viidud kolmandate riikide õigusnormide täitmiseks.
Nii et peab olema koostatud kosmeetikatoodete ohutuse aruanne vastavalt artiklile 10.1 ja lisale 1 milles kirjeldatakse kõik kosmeetikatoote kohta vajalikud andmed arvestades juurde lisatud juhist ja järgides komisjoni otsusega 2013/674/EU kinnitatud juhist ohutuse aruande koostamiseks.
Määruse Artikkel 10 lg 2 kohaselt: I lisa B osas sätestatud kosmeetikatoote ohutushinnangu koostab isik, kellel on diplom või muu ülikooli lõpetamisel antav kvalifikatsiooni tõendav dokument selle kohta, et ta on läbinud teoreetilise ja praktilise koolituse farmaatsia, toksikoloogia, meditsiini või samalaadsel erialal või liikmesriigi poolt võrdväärseks tunnustatud kursuse. Kosmeetikatoodete ohutusehindaja teenust osutavaid ettevõtteid leiate internetist.
Eestis kosmeetikatoodete ohutusehindamisega tegelevad:
1. Meelika Köitjärv; Safety Assessment OÜ; www.safetyassessment.ee ; [email protected] ; telefon: +372 5038878
2. Nadežda Vitkovski: [email protected] ; tel. +37256260118
3. Triin Truu; [email protected] ; Skin Safe OÜ, www.skinsafe.eu, tel +372 5082697
4. Dmitri Vorobjov: NewChem OÜ, [email protected] ; [email protected]; +372 5514378.
Kosmeetikatoodete laboriuuringuid enne kosmeetikatoote turule laskmist saab tootja teha Rahvatervise labori Tallinna laboris ja nakkushaiguste laboris https://www.terviseamet.ee/labor/teenused/kosmeetika-ja-tarbekaupade-analuusid Samal aadressil leiate ka analüüside nimekirja.
Dokumendis http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_190.pdf leiate kosmeetika mikrobioloogilised piirnormid.
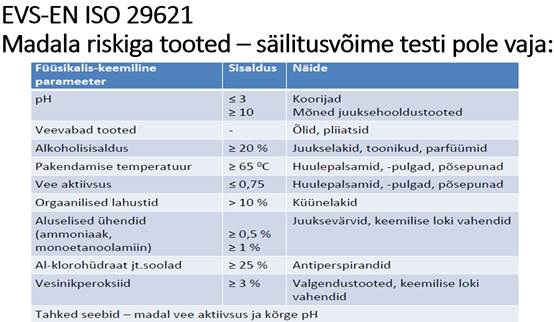
Challenge testi, ehk säilitusvõime testi (antimikroobse kaitse hindamine) ei ole vaja kui kosmeetikatoode ei sisalda vett (nt tükiseep) või muud parameetrid, mis on toodud allpool standardis:
Muud analüüsid olenevad koostisest, näiteks laboriuuring allergeensete lõhnaainete sisaldusele on vajalik kui lisatakse kosmeetikatoodetele eeterlike õlisid ja puuduvad tootjapoolsed spetsifikatsioonid sellele ja allergeensete ainete sisalduse kohta (ühe ja sama nimetusega eeterlik õli võib erineda allergeensete lõhnaainete koguse osas, mis sõltub (erinevus) tingimustest sh kasvupiirkonnast jne ja võib küsida tootjalt et ta annaks sellise spetsifikatsiooni ja allergeenide sisalduse eeterliku õliga kaasa). Säilitusainete sisaldusele test oleneb koostisest (säilitustoorainest) ja kas ja millist säilitusainet lisatakse.
Kindlasti Teil, kui tootjal peavad olema ka füs-kem parameetrid teada (toote pH, tihedus jne). Peab olema tehtud ka stabiilsus test, et teada oma toodete säilimisaega. Mikrobioloogiline test on vajalik. Võimalik et Te oma tootmises hakkate kasutama kiirteste. Keemilised analüüsid olenevad koostisest, nt lõhnaained, säilitusained. Oleneb ka toorainest, nt rasked metallid vms. Tuleb konsulteerida ohutusehindajaga, ta kindlasti aitab.
Kosmeetika tooraineid on kemikaalid ja iga koostisosa kohta peate saama selle tootjalt ohutuskaardi, spetsifikatsiooni ja kasutussoovitused kosmeetikas kasutamiseks, näiteks lõhnakompositsioonide kohta ka veel allergeensete ainete sertifikaadi.
Katsetada tuleb enne kosmeetikatoote turule laskmist ja katsete tulemustest oleneb ka ohutushinnang.
Kosmeetika märgistuse nõuded on toodud kosmeetika määruse https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02009R1223-20240424 art.19.
Koostis märgistusel peab olema ühtses nomenklatuuris - selleks on Teil kasulik kasutada CosIng andmebaasi http://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.simple ning samuti taimede nimed leiduvad Tartu ülikooli taimenimede andmebaasis http://taimenimed.ut.ee/kirjandus.html
Veidi koostisainena kasutatavatest eeterlikest õlidest:
Eeterlikud õlid ise on kemikaalid, puhtal kujul neid nahal ei kasutata ja enamik eeterlikest õlidest on allergiat tekitava toimega. Nii, et nende kasutamine kosmeetikatoodetes peab olema tervisele ohutu, et ei tekitaks kosmeetikatoote kasutamine naha sensibiliseerimist ja ei oleks allergilisi reaktsioone.
Allergeenid, mis on eeterlike õlide koostisosad ja mille esitamine märgistusel on teatud kontsentratsioonis ja tingimustel kohustuslikud on toodud Terviseameti veebilehel aadressil https://www.terviseamet.ee/kemikaalid-igapaevaelus/kosmeetikatooted/koostis#tabel alarubriigis „allergeensed ained“
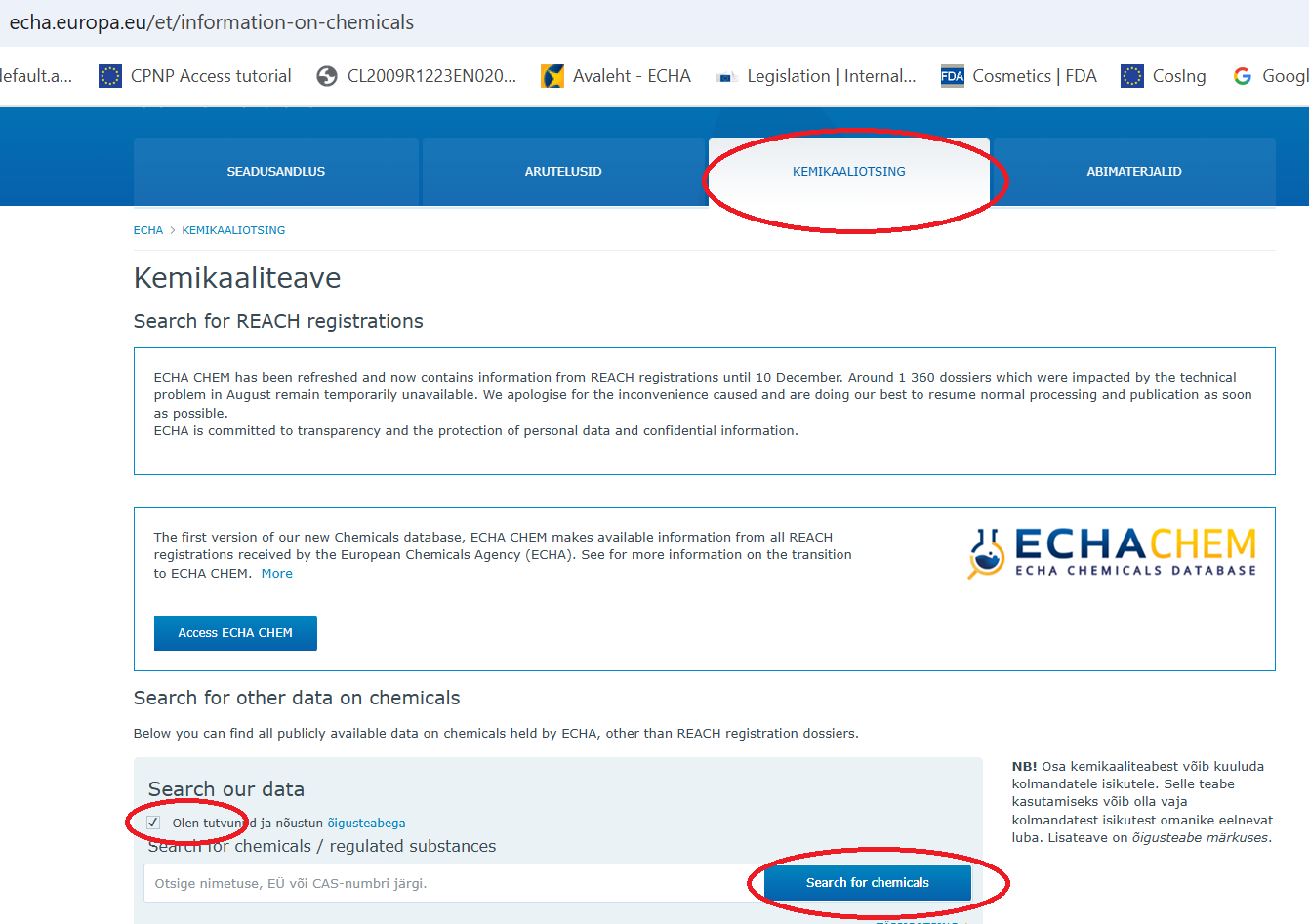
Euroopa Kemikaaliameti (ECHA) veebilehe https://echa.europa.eu/et/information-on-chemicals kaudu saate tutvuda olemasoleva infoga ja teada saada kemikaaliotsingu kaudu kuidas üks või teine aine on klassifitseeritud.
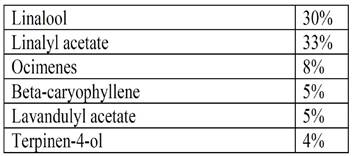
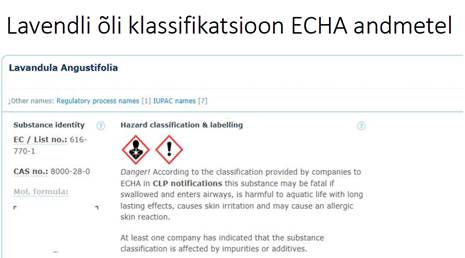
Näiteks lavendliõli sisaldab:
Kuigi kosmeetikatoodetes ei ole lavendliõli kasutamiseks keelustatud, peab olema kindel milline täpne allergeenide kogus lõpptootes on - sellest ja toote iseloomust oleneb ka nende allergeenide väljatoomine eraldi märgistusele (väljavõtte määruse nõudest all pool) et lõpptarbija teaks nende ainete sisaldusest ja juhul kui on ta teadlik, et on allergiline ühe või teise aine suhtes, siis oskaks teadlikult tooteid valida et vältida kokkupuudet nende ainetega, mille suhtes ta allergiline on.
Samuti ei ole lubatud kosmeetikal kasutada meditsiinilisi (sh ravivaid) või biotsiidseid (sh desinfitseerimise kohta) väiteid.
Kosmeetikatoodete tootmisest ja esitatavatest nõuetest lühidalt: tootmisel tuleb järgida hea tootmistava ja täitma kosmeetikamääruses sätestatud koostisele esitatud nõuded, iga kosmeetikatoote kohta peab olema koostatud tooteandmik ja tehtud ohutusaruanne ja ohutusehinnang, kosmeetikatooted peavad olema märgistatud vastavalt art.19 ning teavitatud kosmeetikatoodete CPNP portaalis ning tuleb täita muud asjakohased art. 5 loetletud nõuded.
Lugupidamisega
Marina Karro
teenuse juht
kemikaaliohutuse osakond
+372 794 3530
Terviseamet
+372 794 3500
[email protected]
www.terviseamet.eePaldiski mnt 81, 10614 Tallinn
Käesolev kiri on mõeldud ainult kirja adressaatidele. Kui olete saanud kirja ekslikult, palun teavitage koheselt selle saatjat ning kustutage saadud kiri koos kõikide lisadega. NB! Juurdepääsupiirangu märkega dokumentide avaldamine kõrvalistele isikutele on keelatud.
Saatja: Signe Õunapuu <[email protected]>
Saatmisaeg: kolmapäev, 16. aprill 2025 11:00
Adressaat: [email protected]
Teema: [POTENTIAL PHISHING]Küsimused
Tähelepanu! Tegemist on väljastpoolt asutust saabunud kirjaga. Tundmatu saatja korral palume linke ja faile mitte avada.
Tere
Kirjutan Teile ettevõttest VegePure (S-ilu OÜ). Kirjutan üldmeilile, sest ei oska kellegile konkreetselt kirjutada oma küsimustes.
Esimene küsimus on seoses maale toomisega. Toome maale Ungari ettevõtte CYCLE puhastustooteid. Kuna tooted on nii tõhusad ja head tagasisidet saanud, sooviksin neid rohkem Eestis müüma hakata. Kirjutasin esimesena Grossi Toidukaupadele, kelle sortimenti tooted oma hinna poolest sobiksid. Kuid nad soovisid ohutuskaarte eesti keeles. Muidugi on ohutuskaardid Ungari ettevõttel olemas ja tehtud EL nõuete järgi, aga need on inglise keeles. Kas ma peaksin tõesti kõik need dokumendid eesti keelde tõlkima? Kas on midagi sellega seoses veel vajalik teha?
Lisaks teine küsimus. Kuna olen ka ise juba pikalt endale ja tuttavatele külmpressitud käsitööseepe teinud, siis soovin neid müüma hakata. Mis vajalikud dokumendid jne peaksin ma selleks ära tegema?
Kõike head
--
--
Date: 03.20.2025. v3
1/6
SAFETY DATA SHEET CYCLE TOILET CLEANER LAVENDER-MINT 500 ml, 3000 ml, 10 l
Produced under Regulation 1272/2008/EC and 648/2004/EC
SECTION 1: Identification of the mixture and the company
1.1. Product ID: CYCLE Toilet cleaner lavender-mint
1.2. Identified use: Cleaning of tiles, toilet. Lime scale remover.
User advice: Do not apply on marble and copper or any other acid sensitive surface.
1.3. Manufacturer:
Renew Technologies Hungary Kft.
Address: 1139 Budapest, Lomb u. 15.
Phone: +36 70 474 9900
email: [email protected]
1.4. Emergency telephone:
Health & Toxicology Department in your country
Company phone number in case of emergency (24/7): +36 20 920 4608
SECTION 2: Hazards identification
2.1 Classification of the mixture
2.2. Label elements:
Not a hazardous mixture.
Components: 1-10% Citric acid; 1-10% Non-ionic surfactants, <1% essential oils
Application: Apply undiluted, leave it on the surface for a few minutes, then wipe it off. Do not use on acid-
sensitive surfaces (e.g. marble, copper)! In case of contact with eyes, rinse immediately with water! If using
contact lenses, remove them and continue rinsing!
Storage: in original, sealed packaging, between 10-30 °C, protected from direct sunlight, out of reach of
children.
Best before: 3 years from production. See the date of production on the bottle.
2.3. Other hazards:
PBT: None
vPvB: None
If the mixture is used properly, it does not harm the environment.
The mixture is not fire and explosion hazard.
SECTION 3: Composition data
3.1. Mixture:
Date: 03.20.2025. v3
2/6
Substance/Mixture name
EC number % by weight Classification according to Regulation (EC) No 1272/2008
Spec. conc. limits, M-factors and ATEs
D-Glucopyranose, oligomers, decyl, octyl glycosides, aqueous solution
500-220-1 1-10% - -
Citric acid 201-069-1 1-10% Eye Irrit. 2, H319 STOT SE 3, H335
-
Lavender oil (Natural Oil)
616-770-1 <1% - -
Mentha arvensis extr.
290-058-5 <1% - -
For full text of Hazard and EU Hazard-statements: see SECTION 16
SECTION 4: First aid measures
4.1. Description of first aid measures:
In case of eye contact: In case of contact with the eyes, rinse immediately with water. If using contact
lenses, remove them and continue rinsing.
In case of skin contact: No need for special emergency measures.
In case of ingestion: Never give an unconscious person anything by mouth. The injured person should
drink as much water as she/he can.
4.2. Most important - acute and delayed - symptoms and effects: Not considered to be hazardous.
4.3. The immediate medical attention and special treatment: No need for special treatment. Note to Physician:
Treat according to symptoms.
SECTION 5: Fire-fighting measures
5.1. Extinguishing agent: Normal extinguishing agents used: carbon dioxide, sand, water spray for large fires,
firefighting foam.
5.2. Special hazards arising from the substance or mixture: Not known.
5.3. Advice for fire-fighters: Not known.
SECTION 6: Accidental release measures
6.1. Personal precautions, protective equipment and emergency procedures
For non-emergency personnel: Use personal protection (see in Section 8.)
For emergency responders: Use proper personal protection (see in Section 8.)
6.2. Environmental precautions
Large amounts of spillage materials should not get into the sewage system, water bodies!
6.3. Containment and cleaning up methods and materials
Spills should be neutralized with water. Absorbent material (eg. cloth, fleece) should be used for wiping.
A small amount of product must be removed according to the usual cleaning procedure.
6.4. Reference to other sections: See Section 7. for handling and storage information. For personal protection see
Section 8. Waste management information, see Section 13.
Date: 03.20.2025. v3
3/6
SECTION 7: Handling and storage
7.1. Precautions for safe handling: Contact with eyes and igestion should be avoided.
7.2. Conditions for safe storage, including any incompatibilities: The product must be stored between 10-30℃.
Do not expose it to direct heat or sunlight. No restrictions on storage together with other products. Keep out of reach
of children! Always use the original labelled packaging.
7.3. Product use: Cleaning of tiles, toilet. Lime scale remover.
SECTION 8: Exposure controls/personal protection
8.1. control parameters
No occupational exposure limit for ingredients.
8.2. Exposure monitoring
Respiratory protection: No special requirements
Hand protection: No special requirements
Eye protection: No special requirements
Skin and body protection: No special requirements
Hygiene measures: Handle according to good industrial hygiene and safety practice.
Wash hands before breaks and after work.
Environmental exposure: Does not harm the environment under intended use, transport and storage.
SECTION 9: Physical and chemical properties
9.1. Information on basic physical and chemical properties:
General Information
Appearance (physical state): liquid
Colour: seethrough, opalesque
Smell: flowery, with acidic notes
Important health, safety and environment related information:
pH: 2,0-2,5
Melting point/range: no data
Boiling point: no data
Inflammation point: no data
Flash point: no data
Flammability: not flammable
Explosion hazard: not explosive
Explosion limits: no data
Decomposition temperature: no data
Pour point: no data
Relative density: 1.0-1.1 g/cm3 (at room temperature)
Water solubility: soluble in water
Viscosity: water-like
Oxidizing properties: no data, not typical
9.2. Other information: no data, not typical or not available
Date: 03.20.2025. v3
4/6
SECTION 10: Stability and reactivity
10.1. Reactivity: The mixture is not reactive.
10.2. Chemical stability: At room temperature and normal pressure conditions the mixture is chemically
stable.
10.3. Possibility of hazardous reactions: If used properly, no dangerous reaction takes place.
10.4. Conditions to avoid: Direct sunlight, high temperatures and frost.
10.5. Incompatible materials: Not known.
10.6. Hazardous decomposition products: If used properly, no hazardous decomposition products are formed.
SECTION 11: Toxicological information
11.1. Information on toxicological effects: Product toxicology studies have not been performed, toxicological
assessment made solely on the basis of data on the individual components.
11.1.1. The toxicity data of the ingredients:
Name Acute toxicity,
oral (mg/ttkg)
Acute toxicity, dermal
(mg/ttkg)
Acute toxicity, inhalation
(mg/l)
Citric acid 5400 1 >2000 1 75 2
D-Glucopyranose, oligomers, decyl octyl glycosides
>2000 1 20002 n.d.
Lavender oil >2000 1 >2000 1 20 1
Mint oil >2000 1 >5000 2 n.d.
1 derived from MSDS
2 derived from ECHA database
11.1.2. The symptoms after the exposure of the mixture:
Inhalation: Not known.
Ingestion: Not known.
Skin irritation: Not known.
Eye irritation: Not known.
Sensitivity: Not known.
Sensitization: Not known
Repeated/prolonged exposure: Not known
Carcinogenicity: Not known.
Mutagenesis: Not known
SECTION 12: Ecological information
12.1. Toxicity: Tests have not been performed. The classification and concentration of the ingredients were
determined according to the CLP Regulation requirements and on the basis of toxicological data.
Date: 03.20.2025. v3
5/6
Name EC50 (mg/l)
LC50 (mg/l)
PNEC (g/l)
Citric acid n.d. 440 1 no hazard 2
D-Glucopyranose, oligomers, decyl octyl glycosides
>100 2 126 2 0,176 2
Lavender Oil n.d. n.d. 0,024 2
Mint Oil n.d. n.d. n.d.
1 derived from MSDS 2 derived from ECHA database 12.2. Stability and degradability: biodegradable
12.3. Bioaccumulation potential: no data
12.4. Mobility in soil: it has good solubility and low adsorption capacity in soil.
12.5. PBT assessments: not PBT or vPvB
12.6. Other adverse effects: no data.
SECTION 13: Disposal considerations
13.1. Waste treatment methods: The product residues and wastes must be treated according to the national
regulations of your country.
SECTION 14: Transport Information
The product is according to the control of international transport of dangerous goods agreements (ADR/RID, IMDG,
IATA/ICAO): NOT DANGEROUS.
SECTION 15: Regulatory information
15.1. Safety, health and environmental regulations/legislation for the substance or mixture
Relevant European Community legislation:
CLP Decree: 1272/2008/EC and its amendments; REACH Decree: 1907/2006/EC and its amendments;
Detergent Decree: 648/2004/EC and its amendments
Occupational exposure limits: 91/322/EC Decree and it modification; 2000/39/EC Directive and its
amendments
Workplace safety: 1993. évi XCIII. Act on occupational safety and health; 25/2000. (IX.30.) EüM-SzCsM
Decree about the chemical safety of workplaces; 33/1998. (VI.24.) NM Decree on medical examination and
opinion about job and personal hygiene adequacy; 3/2002. (II.8.) SzCsM-EüM Decree on the minimum health
and safety requirements in workplaces.
Chemical Safety: 2000. évi XXV. Act on chemical safetly and its amendments, certain proceedings related to
hazardous materials and hazardous products furthermore the related activities are regulated in 44/2000.
(XII.27.) EüM Decree and its amendments.
Date: 03.20.2025. v3
6/6
Environment: 1995. évi LIII. Act on the general rules of environmental protection; 2012. évi CLXXXV. Act on
wastes; 98/2001. (VI.15.) Government Decree on activities related to hazardous waste; 72/2013. (VIII.27.)
VM Decree on list of waste
Fire protection: 1996. évi XXXI. Act on fire protection, the technical rescue and fire departments; 54/2014.
(XII.5.) BM Decree on National Fire Protection Regulations
15.2. Chemical safety assessment: No Chemical Safety Assessment has been performed for the mixture.
SECTION 16: Other information
The MSDS is valid for the delivered state of the product. The safety data sheet is only describing the product based
on safety requirements and is not intended to guarantee certain properties and does not replace the product
specifications.
The Material Safety Data Sheet has been compiled according to the best of our knowledge, to the basis of the material
safety data sheets of ingredient manufacturers, the relevant laws, regulations and literature. The rules and regulations
in force are obligatory for the users.
The user is responsible for the appropriate use of the product. The Material Safety Data Sheet does not undertake any
responsibility or legal liability about the use of the product in any circumstances or the results of improper use. The
circumstances of the use (handling, use, storage, disposal, etc) are beyond our competence.
H-phrases of the mixture: None.
H-phrases used in the safety data sheet in Chapter 3.:
H 335: May cause respiratory irritation.
H 319: Causes serious eye irritation.
Do not mix with other chemicals or detergents!
Seosed
| Nimi | K.p. | Δ | Viit | Tüüp | Org | Osapooled |
|---|---|---|---|---|---|---|
| Vastuskiri | 23.05.2025 | 3 | 10.1-1/25/302-4 🔒 | Väljaminev dokument | ta | S-ilu OÜ/ VegePure |