Küsimused
| Dokumendiregister | Ravimiamet |
| Viit | MSO-8/6273-2 |
| Registreeritud | 01.12.2025 |
| Sünkroonitud | 02.12.2025 |
| Liik | Väljaminev kiri |
| Funktsioon | MSO Meditsiiniseadmed |
| Sari | MSO-8 Turujärelevalvega seotud nõustamine |
| Toimik | MSO-8/2025 |
| Juurdepääsupiirang | Avalik |
| Juurdepääsupiirang | |
| Adressaat | Fortrea Development Limited Eesti filiaal |
| Saabumis/saatmisviis | Fortrea Development Limited Eesti filiaal |
| Vastutaja | Piret Põiklik (RA, Meditsiiniseadmete osakond) |
| Originaal | Ava uues aknas |
Failid
From: RA MSO <[email protected]>
Sent: Wed, 26 Nov 2025 05:51:42 +0000
To: "[email protected]" <[email protected]>
Cc: "[email protected]" <[email protected]>
Subject: Vs: Estonia Medical Devices_ Regulatory updates
Dear Sujana Kovvuri,
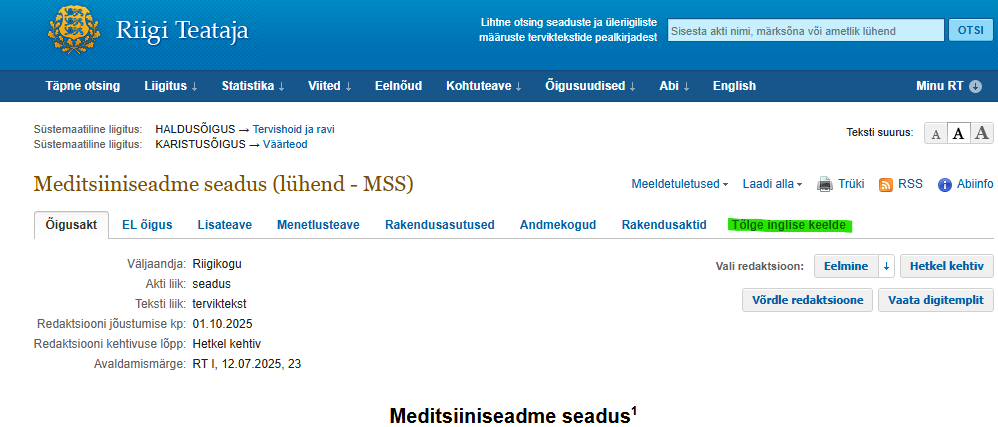
Any regulatory updates can be found at any given moment in Riigi Teataja and specifically when looking at the Estonian Medical Devices Act, you are able to select the version In force as well as move to any previous versions.
To switch to the version in English please choose the tab marked with green:

You can find the latest update time also with the text (marked yellow):

If anyone wants to see what has changed in the regulation, they should choose option „Compare wordings“ and that allows to choose any two versions of the act for side to side comparison. Changes in text will appear in red and green after you have clicked „Compare“.
I will add that the last change in the act did not include changes to safety reporting.
Best regards,
Piret Põiklik
Head of Department
Department of Medical Devices
State Agency of Medicines
[email protected]
+372 5853 3610
1 Nooruse Street
50411 Tartu
Estonia
Phone: +372 737 4140
This e-mail and any attachments transmitted may contain confidential and privileged information. If you are not the intended recipient, please notify the sender immediately by returning the e-mail and permanently deleting what you have recieved. Any dissemination or use of this information by a third person without permission is prohibited and may be illegal.
From: Kovvuri, Sujana <[email protected]>
Sent: Thursday, November 20, 2025 7:23 PM
To: Ravimiamet <[email protected]>
Cc: Madhusudhan, Renuka <[email protected]>
Subject: Estonia Medical Devices_ Regulatory updates
Dear Team,
Hope you are doing well.
As a part of Bi-Annual review, can you please provide us any regulatory updates for Medical devices in Estonia.
We update Safety Reporting Requirements tracker of Medical devices as per the changes required.
Kind regards,
Sujana Kovvuri
Asst II Pss
Patient Safety Solutions
Fortrea Development Ltd
![]()
Fortrea
This e-mail may contain confidential, proprietary, or protected information - including protected health information - that is meant to be viewed only by the intended recipient or company. Any unauthorized review, use, copying, downloading or disclosure is strictly prohibited. If this e-mail is not intended for you, or if you have received this e-mail in error, please notify the sender immediately. Do not forward it to any further recipients and permanently delete this e-mail and all attachments. If you have questions or concerns, please see our privacy policy on Fortrea.com
Seosed
| Nimi | K.p. | Δ | Viit | Tüüp | Org | Osapooled |
|---|---|---|---|---|---|---|
| Küsimused | 01.12.2025 | 1 | MSO-8/6273-1 | Sissetulev kiri | ra | Fortrea Development Limited Eesti filiaal |